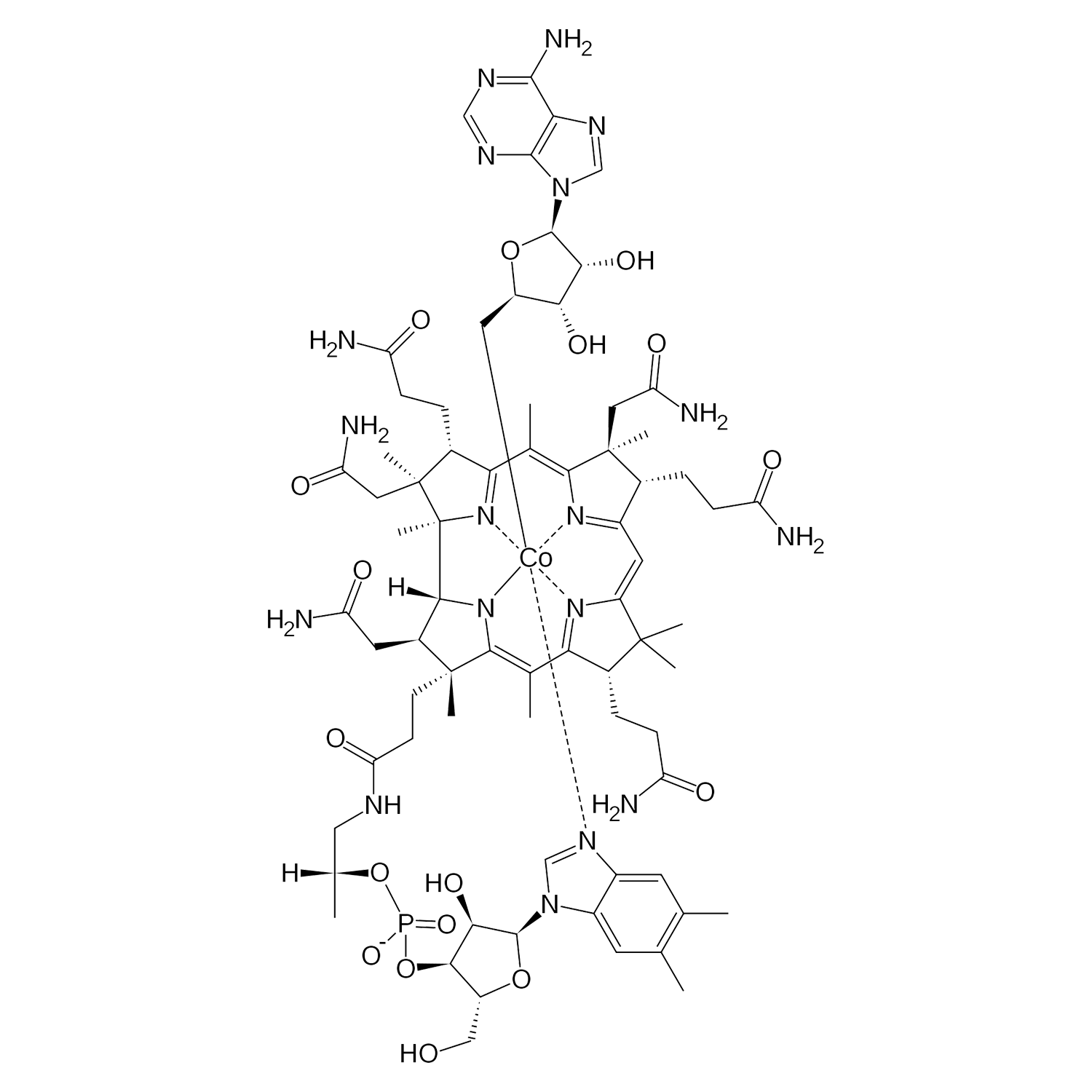
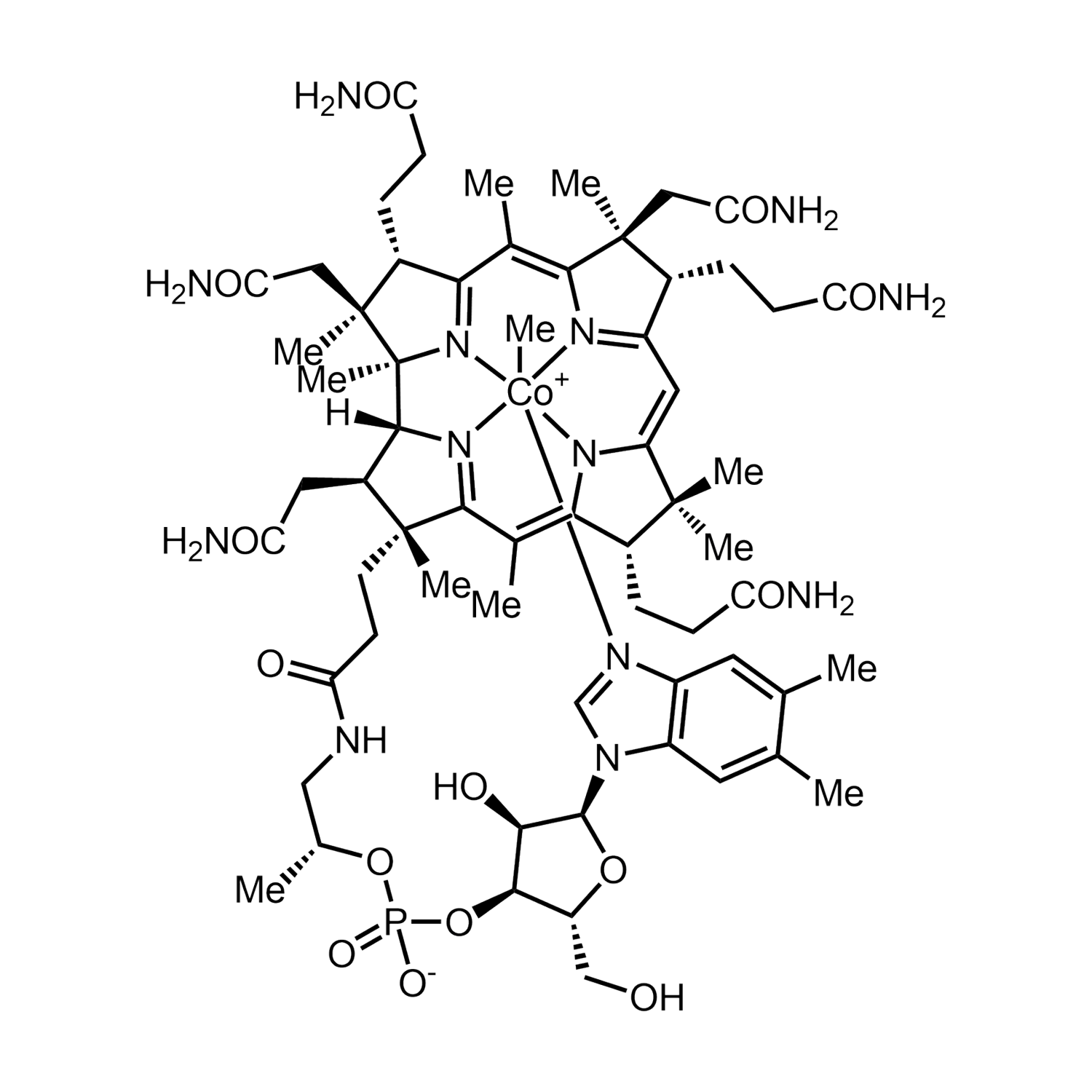
B12 is gemaakt van de biologisch actieve vormen van vitamine B12, methylcobalamine en adenosylcobalamine. Verder bevat B12 organische glycerine, biologisch appelazijn en gezuiverd water. Vitamine B12 zorgt mede voor een goede weerstand en is goed voor het geheugen. De aanbevolen dagelijkse dosering voor volwassenen is 1 ml (20 druppels) per dag.